Guide for authors
1.Aims and scope
2.Review process
3.Manuscript types
4.Manuscript preparation
1. Aims and scope
Outbreak, Surveillance, Investigation & Response (OSIR) Journal offers a free platform for epidemiologic and health sciences study. Our content focuses on various aspects of public health, including, but not limited to:
- Outbreak investigation
- Surveillance evaluation
- Health program evaluation
- Study of public health response, policy and health system
- Heath data analysis
- Systematic reviews
- Other public health research
2. Review process
Initial review
Upon submission, articles are scrutinized for plagiarism via ThaiJo systems, while the journal managers ensure that the articles conform to the submission preparation checklist with regard to formatting and clearance. The content of the articles is then subjected to a brief double-blind review by the Chief Editor, and one of the Senior Editors who is not listed as an author. Manuscripts that do not adhere to the prescribed format or are partly/wholly incomprehensible text will be returned to the authors for revision. Manuscripts that are outside the scope of the journal or deemed to be in violation of ethical standards will be rejected (Figure 1).
Double-blinded peer-reviewed
Once an article is initially accepted by the Chief Editor, it then proceeds to a full double-blind peer review process. The journal manager chooses two expert reviewers who are not authors and are not affiliated with any of the corresponding author's institutions or divisions. The reviewers' comments and initial decision are sent to the corresponding author who is then given a chance to revise the article. The same Chief Editor determines if the revised article should then be accepted or rejected and may provide comments for improving the manuscript. One reviewer will assist the author to improve the quality of the article. After the manuscript's quality is approved by the reviewer, the Chief Editor provides the final decision on whether to approve the manuscript for publication (Figure 1).
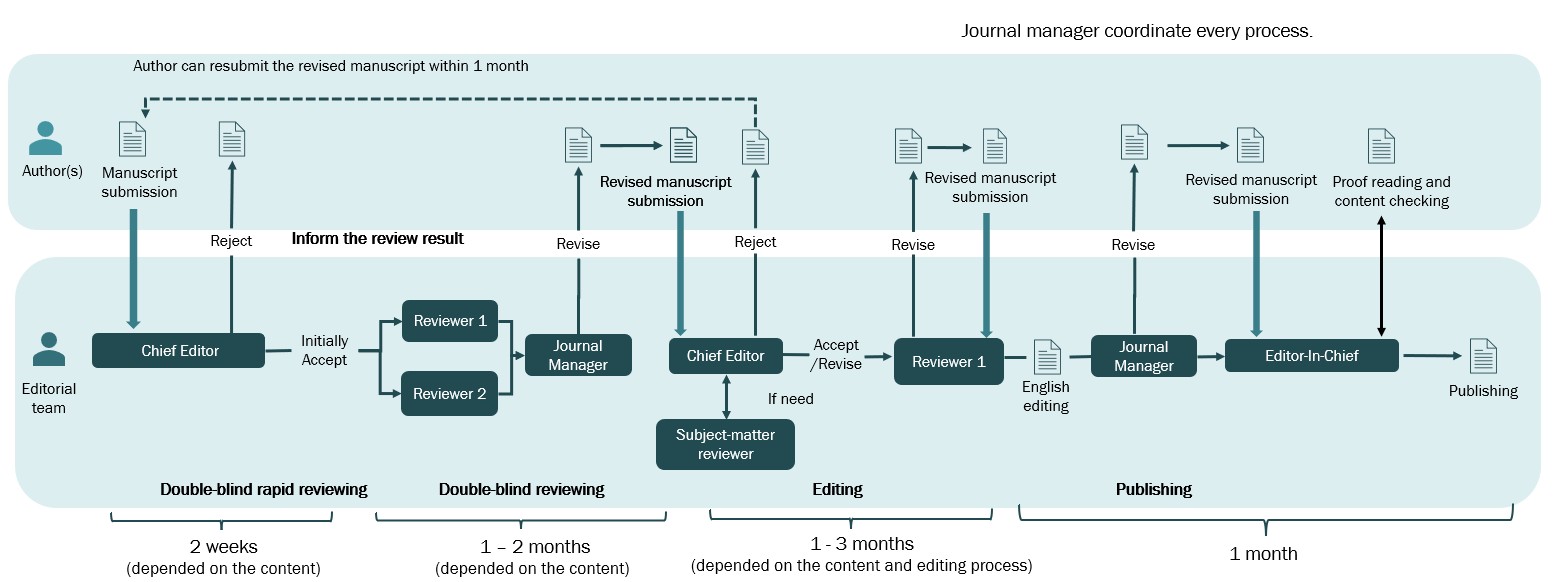
Figure 1. Review and publication flow of the Outbreak, Surveillance, Investigation & Response Journal
3. Manuscript types
Outbreak, Surveillance, Investigation & Response (OSIR) Journal publishes four types of articles, including invited editorials, review articles, original research articles, and systematic reviews.
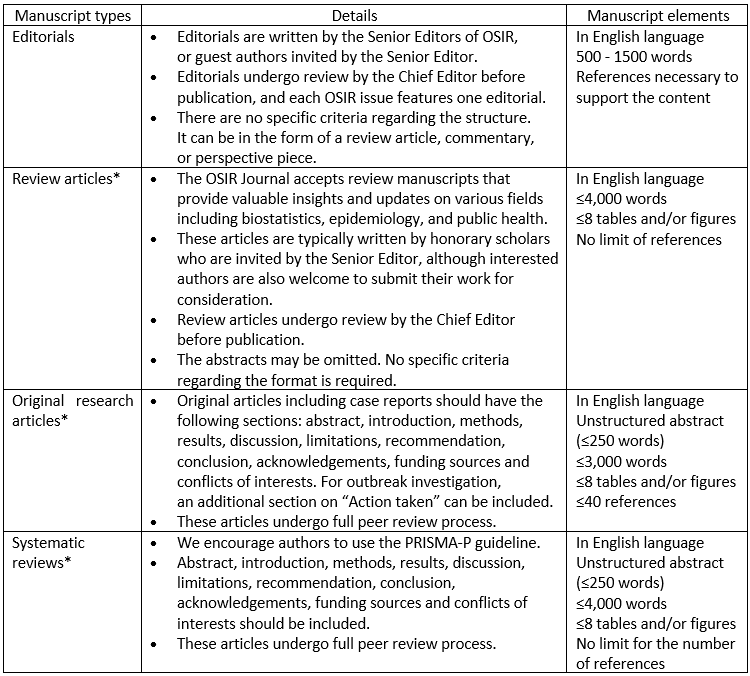
Note: *If an author is invited by a Senior Editor, their article will be published in the “Invited article” section of the journal.
4. Manuscript preparation
The manuscript must not have been submitted or published in any peer-reviewed journal.
All co-authors have given their approval for the manuscript to be published. The manuscript has also been approved by all relevant organizations/institutions for publication. The author and supervisor (if applicable) responsible for dissemination of information in the manuscript must sign the OSIR clearance form and submit it to complete the submission process.
Datasets related to the information in manuscript should be professionally prepared and be made available upon request as authors may be asked to check the data during the editing and reviewing processes.
Manuscripts submitted for OSIR publication must be in Microsoft Word file format and uploaded on the ThaiJo submission system.
The text should be in Calibri font size 12 and double-spaced.
Manuscript formatting
4.1 Title
The title should describe the study or pose a question that expresses the primary objective. It should include the disease or event, the time and place it occurred.
4.2 Authors, affiliations, and corresponding author
The names of all authors, including their middle name(s) and surname, along with their affiliated institutions must be listed. The corresponding author should be indicated with an asterisk and their email address should be provided.
4.3 Abstract
The abstract should be non-structured and must not exceed 250 words in length. This word count does not include the title, author list, information in the heading and keywords.
4.4 Keywords
The keywords should be listed below the abstract and a minimum of three words is required.
4.5 Introduction
This section describes why you conducted your study. The following are the topics suggested for the introduction.
Context
- General information about the significance of the disease
- Occurrence of disease in region
- Surveillance data or other information on disease burden and risk factors (susceptible population)
- Worldwide magnitude of disease (number of cases, rank on scale of morbidity/mortality)
- Regional/National magnitude
- Provincial magnitude
- Historical perspective (disease trend, emerging or re-emerging)
- Typical demographics of cases
- Prevention and control strategies currently in use (i.e., vaccination, vector control, etc.)
- Availability and type of diagnostic testing
Biological information (extensive detail is not necessary)
- Microbiology/pathophysiology
- Natural history of an infection
- Clinical presentation of infection
- Seasonality of the disease
- Mode of transmission
- Reservoirs
Gap in knowledge that made this work necessary
- Information that is currently missing and the reason the study was conducted.
- Importance of the research question
- Impact of the research
Objective(s)
- Verification of an outbreak
- Determination of disease’s etiology
- Risk factors of a disease
- Determination/effectiveness of control measures
- Route of transmission
- Cite evidence that supports, refutes, or questions related hypotheses
- To describe the natural history of a disease
4.6 Methods
This section describes how you conducted your study. This section would provide enough information to allow someone in a similar situation to replicate exactly what you did. The following points provide a guide:.
Location and timeframe
- Map of the province and country
- Urban vs. rural setting
- Population of study area
- Unique characteristics of population and geography
- Duration of the study and dates of initiation, completion, other relevant dates
- Date in yearly format (i.e., 8 November 2023)
Study population
- Case definition
- Case identification and recruitment
Study design
- Descriptive
- Case-control
- Cohort
- Cross-sectional
- Ecologic
Data collection
- Data source and tools
- Type of interview
- Biological/chemical samples
- Environmental samples
Analysis
- Type of analysis
- Statistical tests and software program used
- License of the software program, if it is not freeware
- Reference for open-source software
- Significance level
Ethics
- Ethical approval acquired from the related organization and approval number (if any).
- Ethics statement
4.7 Results
This section describes what your study found. Key results from the analyses which support the conclusion and recommendation should be reported. We strongly encourage using tables and figures to display your findings. Below is a guideline of how to report the results.
Demographic
- Basic demographic characteristics of the sample
Tables/figures
- Tables and figures should be simple, clear and easily understandable
- Avoid pie charts and 3-D graphics
- Describe the main pattern and features without repeating all the raw data from each table/figure
- For more information see Edward Tufte’s books: <http://www.edwardtufte.com/tufte/books_visex>.
- For creating Epi Curves, please follow the CDC Guidance: <https://www.cdc.gov/training/quicklearns/createepi/>
- Titles of tables and figures should be shown above the tables and below the figures.
- The tables and figures should be listed in Arabic numerals cited in the Text (i.e., Table 1)
- The tables and figures should be placed after the reference section
- The figures in editable format should be attached in separate files during submission
Response rate
- Were you able to obtain information from most of your cases?
- Define the denominator
Characteristics of cases/sample population
- Describe by person, place and time
Primary outcome
- Findings of the primary analysis you proposed in your methods section and findings that address the objective of your work with descriptive statistics
- Measures of association (univariate and/or multivariate analyses) and inferential statistics (confidence intervals or p-value)
Laboratory results
- Type of sample
- Laboratory test used
- Reference level
- Sensitivity and specificity of laboratory tests
- Reference for laboratory method
Further analysis
- Secondary analysis
- Environmental results
4.8 Action taken
This section should be included in an outbreak investigation manuscript. It provides a detailed account of the measures, interventions, or actions implemented in response to the outbreak under study. It serves as a critical component of the manuscript as it outlines the practical steps taken to manage and control the outbreak.
4.9 Discussion
This section explains the findings of your study. You should not repeat your findings here - just refer to them as needed to discuss them.
- Summarize the key findings
- Linkage of overall results with the objectives
- Interpret each of your results
- Compare your findings with the literature. Agree or disagree? If disagree, explain why
- Significance of findings
4.10 Limitations
This section addresses aspects of the data or analysis that might affect the accuracy or validity of the results. The cause, consequences and how you might mitigate its effect should be provided.
4.11 Recommendations
This section should include practical information to the readers and where the work can have the greatest impact on controlling the disease or on public health. The results and discussion must support the action and recommendation.
4.12 Conclusion
The author should conclude the results and recommendations following the objectives stated in the manuscript.
4.13 Acknowledgements
The author should acknowledge anyone or any organizations that provided assistance in their work. The acknowledged person(s) should not be on the author list of the manuscript.
4.14 Funding sources
The author should state the funding source if they received any. The name(s) of the funding organization(s), and the use of funds should be listed.
4.15 Conflicts of interests
The author should declare any conflicts of interest related to their work in the manuscript. There should be no conflict of interest.
4.16 References
The references must be written in the Vancouver style and the URLs for the references should be provided, if available. The reference must be cited by consecutive numbers in the text. The cited number and reference must match. For more details, please see the guidance on Vancouver style: https://www.nlm.nih.gov/bsd/uniform_requirements.html.
4.17 Others
Abbreviations
The use of abbreviations should be kept to a minimum. If the term is referred to no more than three times, the abbreviation should not be used. In both the abstract and the body of the text, the full term should be written first, followed by the abbreviation enclosed in parentheses. The abbreviation must then be used consistently.
Names of organisms
The genus and species of all organisms should be italicized and written in full terms on first use. The genus can then be abbreviated to the standard name. However, if the author refers to another species, the full term should still be written and should follow the previous statement.
Footnotes
All authors should use the following footnote symbols in sequential order: *, †, §, ¶, **, ††, §§, ¶¶, etc. Except for the asterisk, all symbols should be presented in superscript. Footnote symbols should be placed outside of any punctuation except for semicolons and colons.